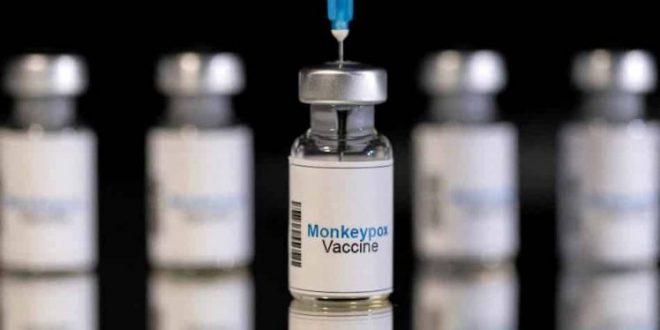
The European Medicines Agency (EMA) on Friday said it approved the use of a human smallpox vaccine to expand its use against the spread of monkeypox.
“The EMA’s Committee for Medicinal Products for Human Use (CHMP) has recommended extending the indication for the smallpox vaccine Imvanex to include the protection of adults against monkeypox,” the European regulator said in a statement.
This vaccine from the Danish company Bavarian Nordic has been used in the European Union since 2013. It was approved because of the similarities between these two types of smallpox.
Several countries of the European Union, including France, had already ordered this vaccine in May.
The epidemic situation has worsened in recent weeks, with more than 15,300 cases now recorded in 71 countries, according to the latest figures from the United States health authorities (CDC), the most up-to-date.
 AfricaTopSuccess They make Africa
AfricaTopSuccess They make Africa